Tel: +86-187 0254 3408
Email: sales@zjzsbiotech.com
WhatsAPP: +86-187 0254 3408

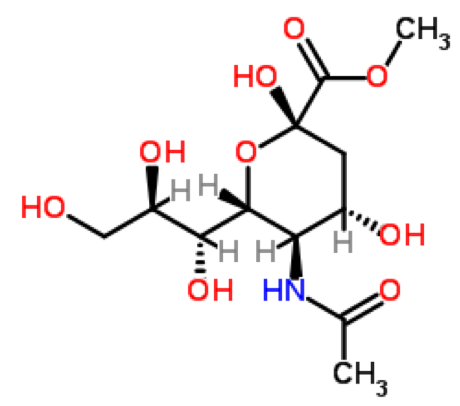


| Chemical Name | N-Acetylneuraminic acid methyl ester |
| Synonyms |
Methyl 5-Acetamido-3 5-dideoxy-beta-D-glycero-D-galacto-2-nonulopyranosylonate
|
| CasNo. | 50998-13-5 |
| Molecular Formula | C12H21NO9 |
| Molecular Weight | 323.30 |
| Appearance | White Powder |
| Storage and Shipping Conditions | Cool and dry |
| Industry | Cosmetic raw materials pharmaceutical intermediates |
| Applications | Zanamivir intermediate NANA |
| Functional Properties | N-Acetylneuraminic Acid (Neu5Ac) is a type of sialic acid (Sia). Acts as the terminal sugar in various biological molecules, including cell surface glycans, glycoconjugates, oligosaccharides, lipo-oligosaccharides, and polysaccharides. Sialic acids, including N-Acetylneuraminic acid, are involved in various physiological functions, emphasizing their importance in human health. |
|
Uses |
Given its role in essential biological processes, N-Acetylneuraminic acid and its derivatives may have potential applications in medicine, biochemistry, and pharmacology. |
InChI:InChI=1/C12H21NO9.H2O/c1-5(15)13-8-6(16)3-12(20,11(19)21-2)22-10(8)9(18)7(17)4-14;/h6-10,14,16-18,20H,3-4H2,1-2H3,(H,13,15);1H2
The literature on the acetylation of the methyl ester of N-acetylneuraminic acid (1) is confusing and this note is intended to clarify the situation. In 1966, Kuhn et al.’ reported that acetylation of 1 with acetic anhydride in the presence of a catalytic amount of aqueous 60% perchloric acid at 40” for 2.5 h gave 61% of crystalline methyl 5-acetamido-2,4,7,8,9-penta-O-acetyl-3,5-dideoxyD-glycero-o-gakzcfo-2-nonulopyranosonate (m.p. 156-157”).
The 8-carbon analogue of N-acetylneuraminic acid was synthesized by the alkaline condensation of 2-acetamido-2-deoxy-d-lyxose and di-tert-butyloxaloacetate.…